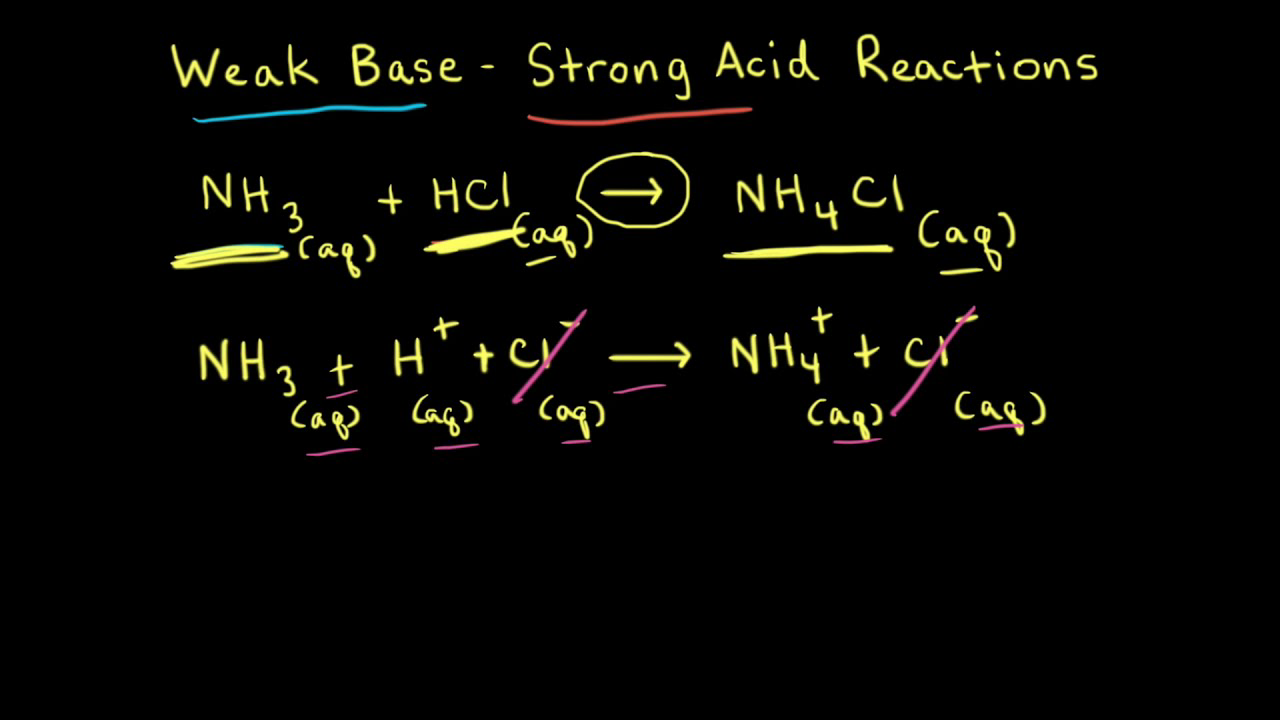
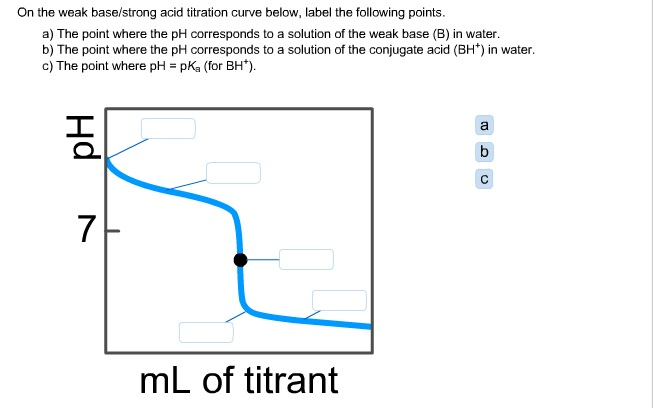
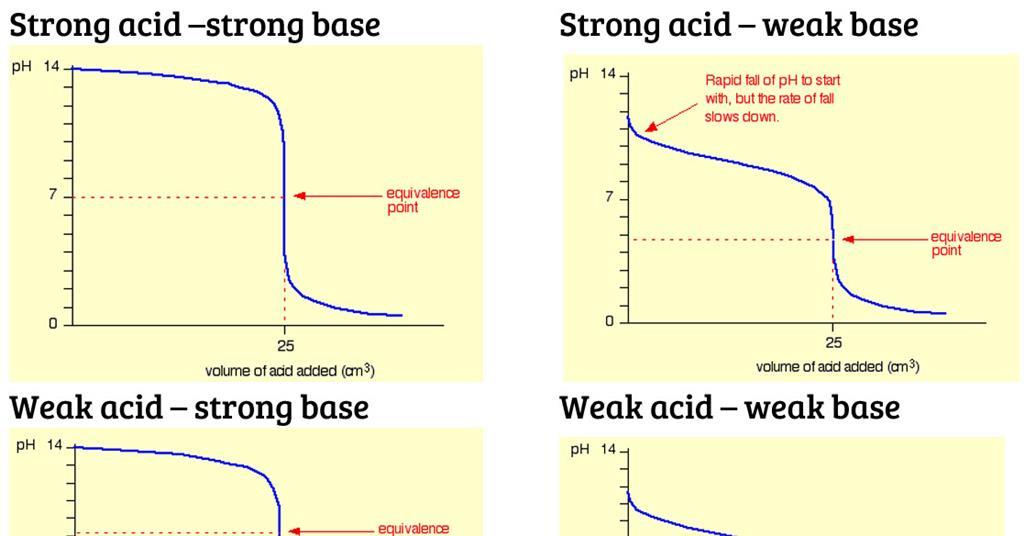
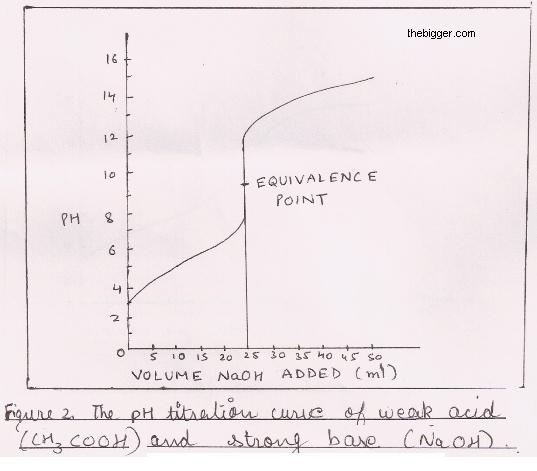
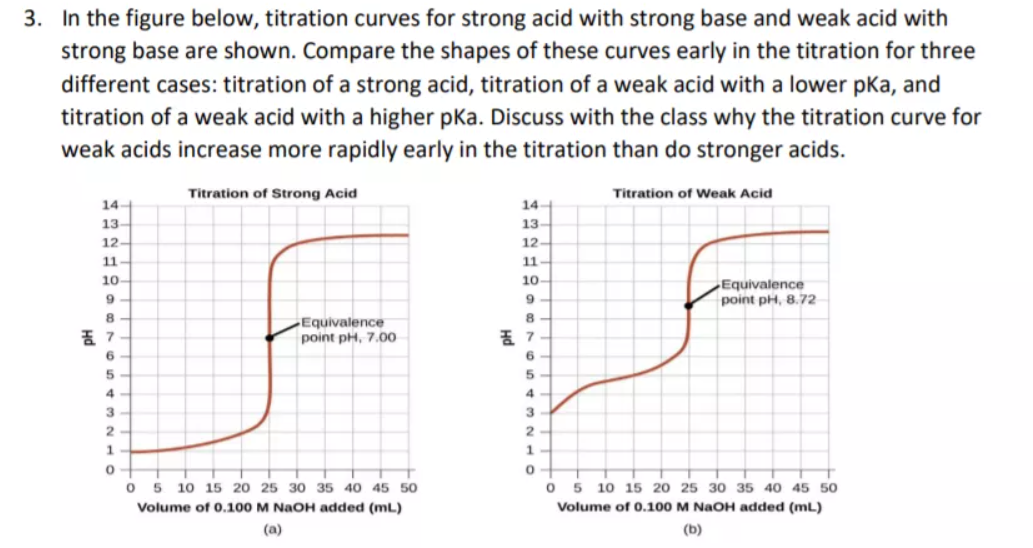
18.pptx - Mixtures of Solutions Weak Acid - Weak Base K depends on the K's of the reacting species. K = Ka(reacting acid) /Ka(produced acid) The | Course Hero
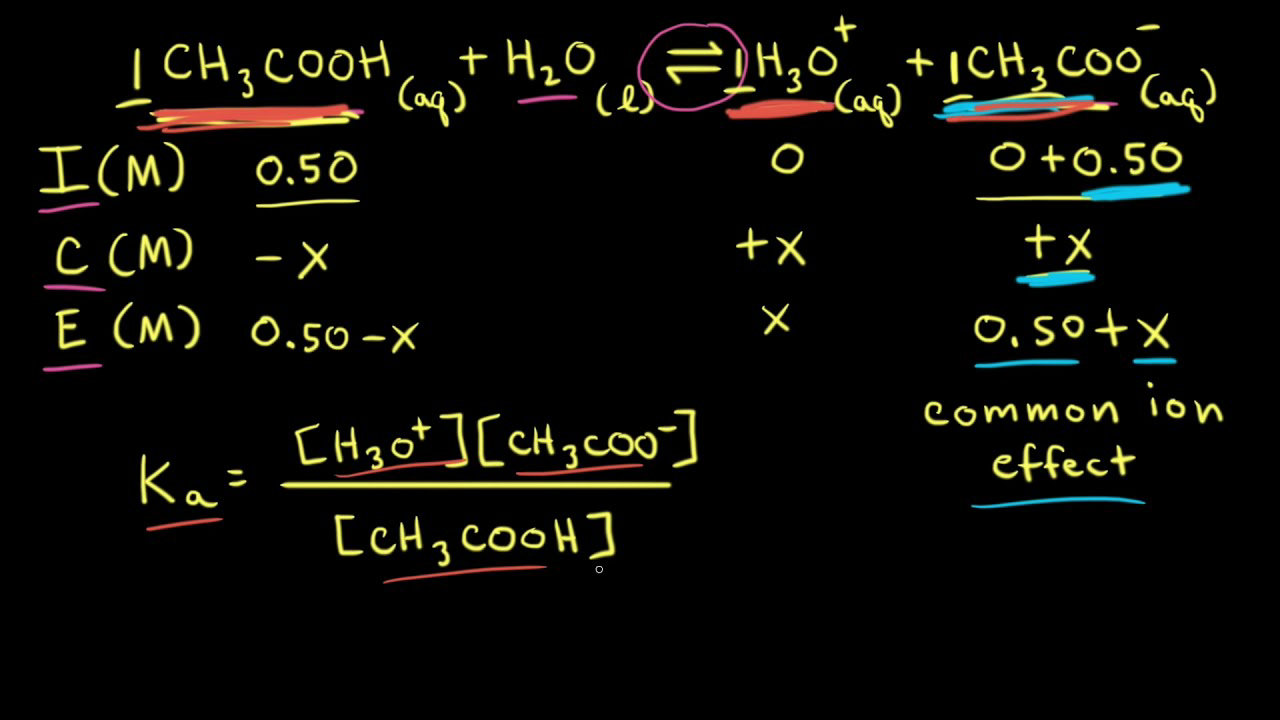
Worked example: Calculating the pH after a weak acid–strong base reaction (excess acid) (video) | Khan Academy

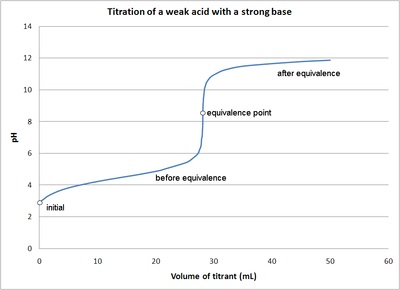
ph - What is causing the buffer region in a weak acid - strong base titration? - Chemistry Stack Exchange
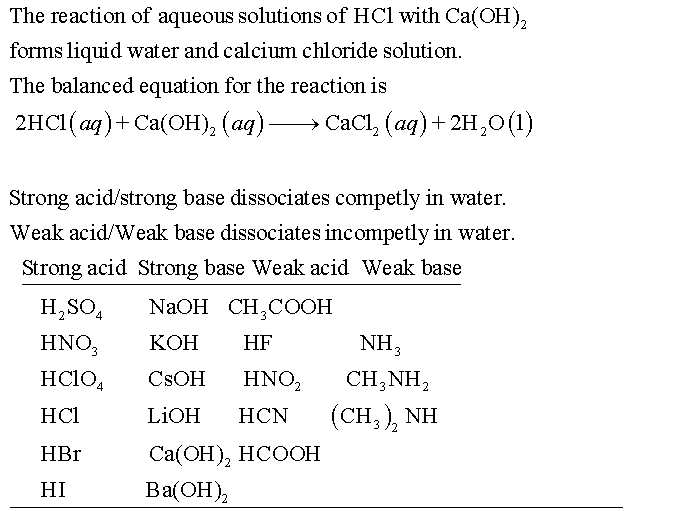
Classify each substance as a strong acid, strong base, weak acid, or weak base? - Home Work Help - Learn CBSE Forum
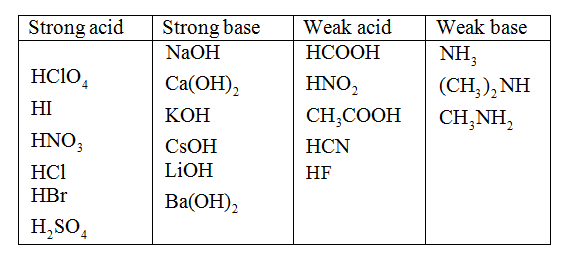
Classify each substance as a strong acid, strong base, weak acid, or weak base - Home Work Help - Learn CBSE Forum
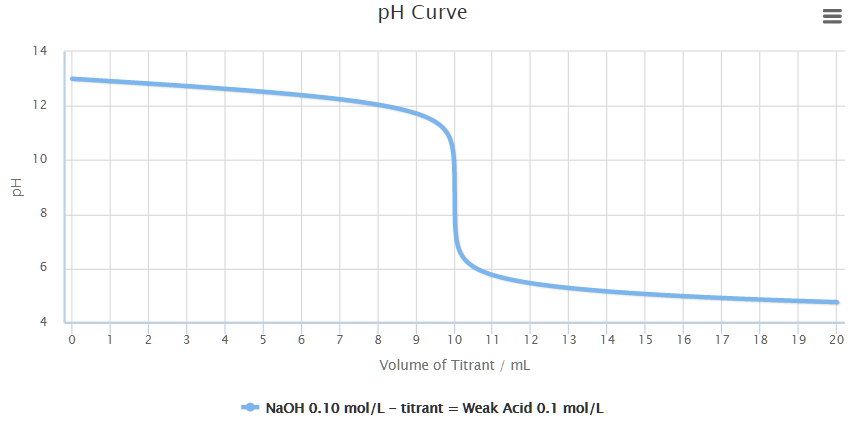
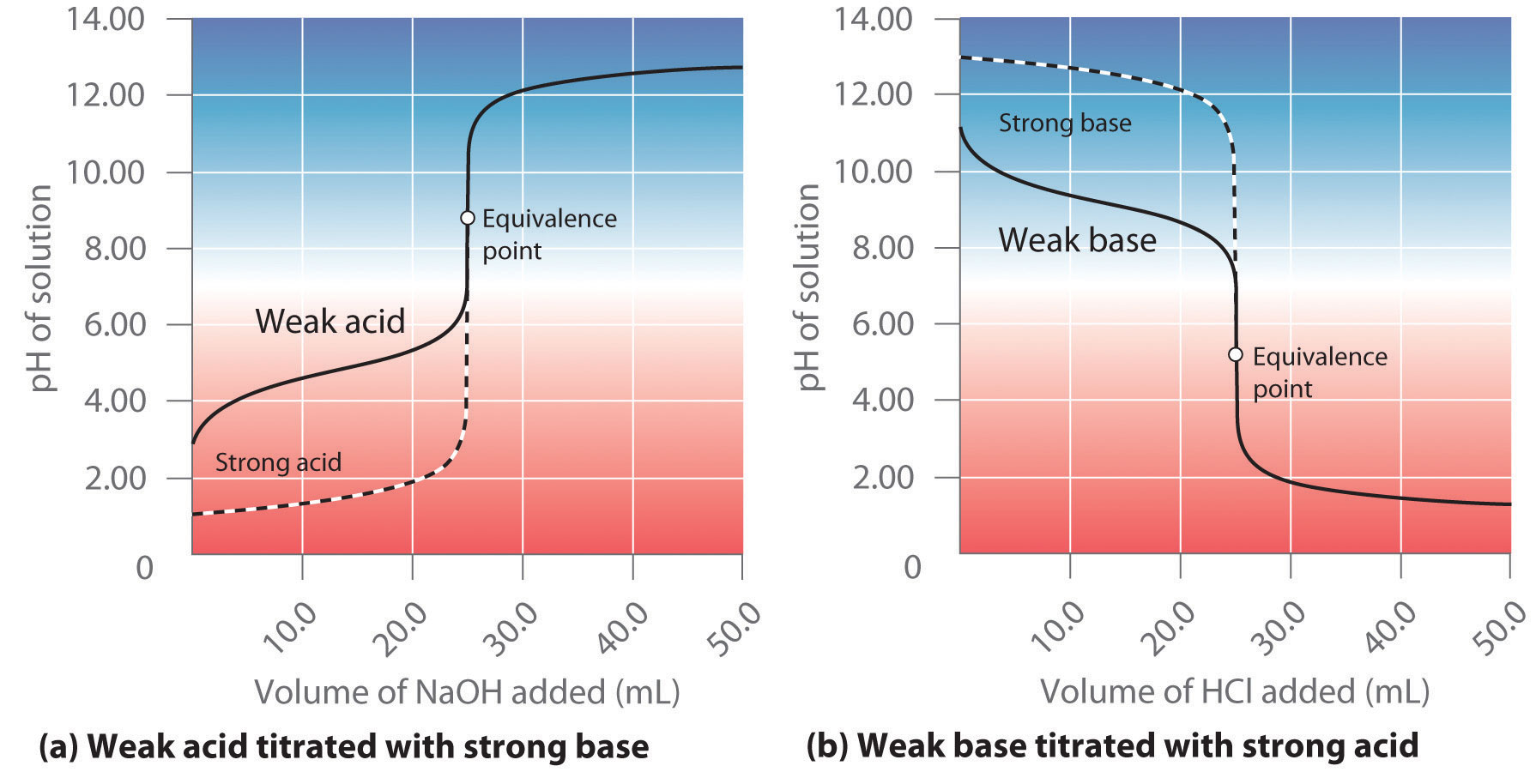
While performing the titration of a weak acid and strong base, can we put weak acid in the strong base rather than the usual strong acid in a weak base? | Socratic
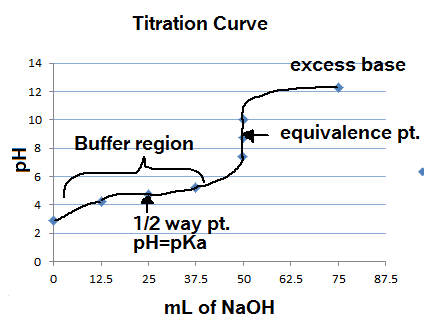
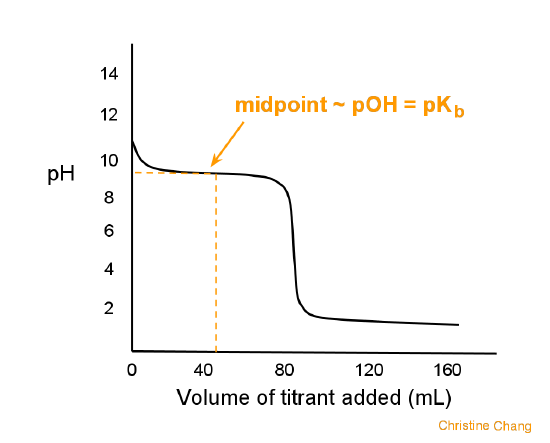
What considerations apply when a weak acid is titrated with a strong base, as opposed to a strong acid with a strong base? | Socratic